
“These results are impressive and clinically meaningful,” wrote Daniel F. It will take longer follow-up to determine whether T-DM1 will ultimately improve how long patients live overall, Dr.
#T DM1 CLINICAL TRIALS TRIAL#
The researchers who led the trial estimated that, at 3 years after beginning adjuvant treatment, 88% of women treated with T-DM1 were alive and free of invasive cancer, compared with 77% of women treated with trastuzumab. Participants in the KATHERINE trial were randomly assigned to receive adjuvant therapy with either T-DM1 or trastuzumab (in 3-week treatment cycles for up to 14 cycles). Studies have consistently shown that women with early-stage breast cancer -particularly those with triple-negative or HER2-positive disease -who don’t have residual disease after neoadjuvant chemotherapy live longer without their disease recurring, compared with women who have residual invasive cancer.Īdjuvant therapy with trastuzumab has been a standard treatment for women with HER2-positive breast cancer, regardless of whether they have residual disease. Some women with very small cancers, however, may proceed straight to surgery, she said.įor many women, neoadjuvant chemotherapy will eliminate all evidence of residual disease, Dr. The goal of neoadjuvant therapy is to eliminate as much cancer as possible prior to surgery, and many women with early-stage HER2-positive breast cancer now receive neoadjuvant therapy, explained Janice Lyons, M.D., a radiation oncologist at the Case Comprehensive Cancer Center in Cleveland, who specializes in treating breast cancer. Roughly 20% of the women also received pertuzumab (Perjeta) as part of their neoadjuvant therapy. All women in the trial had evidence of residual disease after neoadjuvant therapy, which included chemotherapy and trastuzumab. The KATHERINE trial-funded by the manufacturer of T-DM1, Genentech-enrolled nearly 1,500 women with early-stage HER2-positive breast cancer, meaning their cancer was confined to the breast and the axillary lymph nodes.
Once the antibody binds to HER2 on cancer cells, emtansine is released into the cells.Īfter showing that T-DM1 improved how long women with metastatic HER2-positive breast cancer live, researchers quickly moved to test the drug in women with early-stage disease.


The antibody portion of T-DM1, in addition to blocking the activity of the HER2 protein on cancer cells, serves as a homing device for emtansine. Known as an antibody–drug conjugate, T-DM1 chemically links the trastuzumab antibody to the chemotherapy drug emtansine (also known as DM1). Trastuzumab latches on to HER2 proteins on the surface of breast cancer cells and prevents HER2 from stimulating cancer cell growth. Trastuzumab, a monoclonal antibody, was among the first FDA-approved targeted cancer therapies and has long been an established therapy for HER2-positive breast cancer. “T-DM1 has now become the standard of care for women with HER2-positive breast cancer and residual invasive cancer following neoadjuvant therapy,” he said. The trial results, and the subsequent FDA approval, have already had an important impact on patient care, Dr. Geyer said, “the majority of women tolerated the drug reasonably well.”
#T DM1 CLINICAL TRIALS FULL#
As a result, more women taking T-DM1 (29%) did not complete the full course of the adjuvant treatment than women taking trastuzumab (19%).īut many of these women did not have to stop taking the drug until they were near the end of their adjuvant treatment period, explained the study’s lead investigator, Charles Geyer, Jr., M.D., of the Virginia Commonwealth University Massey Cancer Center.
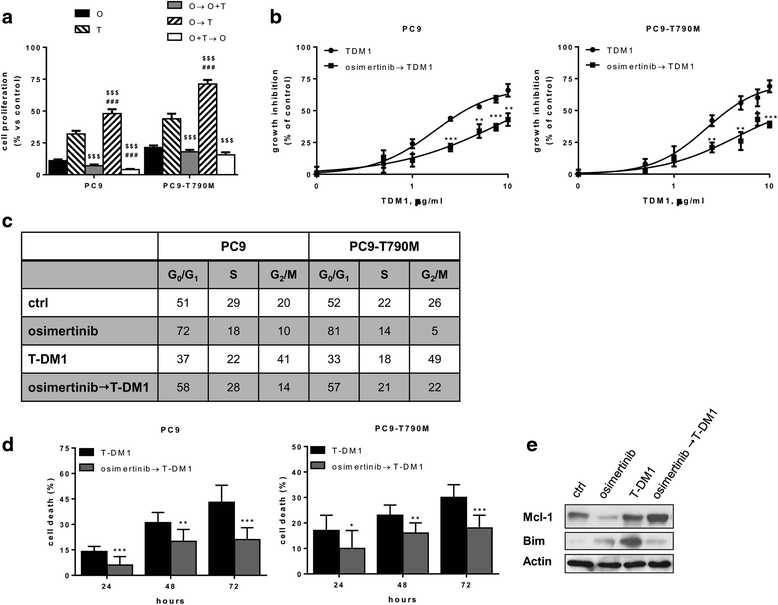
Side effects, including serious side effects, were more frequent in women treated with T-DM1. In the trial, women treated with T-DM1 had a 50% reduced risk of their cancer returning or death than women treated with trastuzumab. The new approval, announced on May 3, is based on findings from a large clinical trial called KATHERINE that compared T-DM1 with trastuzumab (Herceptin) as an adjuvant treatment. However, to be eligible to receive the drug under this newly approved use, women must first have undergone presurgical, or neoadjuvant, therapy to shrink their tumors and still have some signs of remaining invasive cancer, called residual cancer, in the breast or nearby lymph nodes. Under the expanded approval, it can now be used when the cancer is far less advanced: as a post-surgical, or adjuvant, treatment in women with early-stage HER2-positive breast cancer. The Food and Drug Administration (FDA) has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla) to treat some women with HER2-positive breast cancer.Īdo-trastuzumab, also called T-DM1, was initially approved by FDA more than 6 years ago to treat women with metastatic HER2-positive breast cancer.


 0 kommentar(er)
0 kommentar(er)
